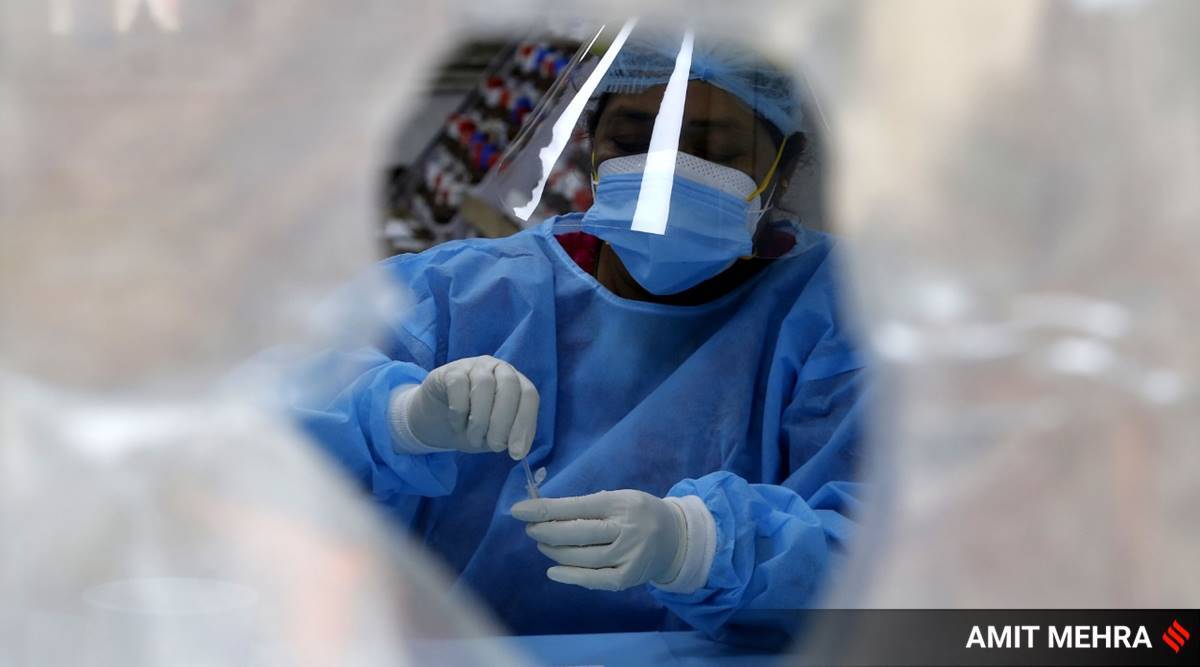
India needs a model of consolidated and pooled procurement to drive down testing costs, improve the quality of testing, and increase test kit availability
Written by Manisha Bhinge and Sarang Deo
India is braving an unprecedented storm with the second wave of Covid-19 sweeping through the country. Health systems are overwhelmed and the need for critical medical supplies is expanding by the minute. While the liberalised vaccination programme offers hope, the significant vaccine shortage and multiple variants of the virus have our collective guards up. Amid rising cases, testing — a potent tool to contain the spread — is escalating steadily and inching close to two million tests daily.
The government’s emphasis on reverse transcription-polymerase chain reaction (RT-PCR), considered the gold standard of tests and essential for sequencing purposes, also continues to remain high.
The appetite for testing among populations has risen in this wave. Diagnostic labs are reeling under the stress of skyrocketing demand and some private labs have called for government intervention to ensure seamless and expeditious supply of critical material (reagents for RT-PCR). However, with parallel tasks of purchasing vaccines under the liberalised Covid-19 vaccination programme, securing medical oxygen supplies, and managing hospital beds, state governments are stretched thin on resources including financial resources. At this critical juncture, pivoting to cost rationalisation approaches holds the key to sustaining the pandemic response momentum.
Throughout the pandemic, governments have banked on the private sector to augment Covid-19 testing due to limited capacity in the public sector. But with the ongoing wave increasingly afflicting rural populations, there is a renewed need to review the costs attached to testing.
For marginalised and underserved pockets that hail typically from the lowest socioeconomic strata, even state-imposed price caps trigger affordability constraints and lock them out of accessing care in the private sector. And in geographies where the private sector serves close to 70 per cent of the healthcare needs, such inaccessibility has a staggering impact on health outcomes and widens the gulf of inequity. Thus, the affordability of testing is a cornerstone of equitable pandemic response.
These state government-imposed ceiling prices on the private sector reflect a clear intention of making testing accessible and affordable for all populations. However, these price caps fail to account for the diverse cost elements: Consumables, personnel, transportation, overheads.
This essentially handcuffs the private sector from expanding testing facilities. A study conducted by the Max Institute of Healthcare Management at the Indian School of Business (ISB), supported by The Rockefeller Foundation, identified that collection and testing consumables (viral transport media kit, reagents, testing kits, plate) constitute around 88 per cent of the total cost of the RT-PCR test. This implies that reducing the procurement cost of consumables can slash the overall cost of tests, leading to lower test prices for consumers.
The study suggests a model of consolidated and pooled procurement to drive down testing costs, improve the quality of testing, and increase test kit availability. Substituting fragmented buyers – governments and private labs – with a consortium can lead to a three-fold cost reduction. With greater bargaining power, the consortium can leverage volume purchase to negotiate with different sellers and ensure a uniform and lower cost for all buyers. If this mechanism is facilitated by the central government, in addition to test prices, information exchange across states can also be standardised, to minimise existing disparities.
The Global Fund to fight Malaria, AIDS and TB uses the pooled procurement mechanism to reduce the cost of insecticide-treated malaria nets and antimalarial treatment to less than $2 and $0.58, respectively. Closer to home, the National AIDS Control Organisation (NACO) and Central TB Division (CTD) have, in the past, piloted similar exercises and their functional expertise can be effectively harnessed for Covid-19 testing cost reduction.
Private actors have even partnered in the absence of government intervention to catalyse such strategies. Most notably, under the TB programme, private laboratories formed the Initiative for Promoting Affordable and Quality TB Tests (IPAQT) alliance in 2013 and successfully negotiated with diagnostics manufacturers to lower prices for equipment and reagents. Consequently, not only did the cost of TB testing reduce for patients, but it also generated greater testing demand.
Elaborate and accurate test demand forecasting channels have proven to be a key ingredient in pooled procurement strategies. It enables manufacturers to meticulously plan their production, pre-empt shortages, and avoid wastage, thereby reducing cost across the value chain.
For procurement of RT-PCR consumables, the vehicle may differ from IPAQT. Modifications would be needed to account for greater test kit volumes, a higher number of suppliers and buyers in the market, as well as quality considerations.
This refashioned model can propel the country’s Covid-19 testing framework towards greater overall capacity, access, and equity. Extending the benefits of pooled procurement to private players can translate to affordable and increased testing options for the public. It will also serve as an incentive for private labs to join the Indian Council of Medical Research’s (ICMR) laboratory network and scale up testing.
As we tide over the current ordeal, it is critical to remember that intensified testing is not a substitute for the vaccination programme, and vice-versa. While universal vaccination forges a barricade against the virus, it cannot be the only answer as building that barricade amid a vaccine shortage will take time.
Testing is essential, as it is testing data that provides real-time information on a virus that continues to reinvent itself. These are distinct tools that complement each other and with strategic calculation and smart execution, they can be deployed to transform the challenging landscape we face and prepare for future infectious disease pandemics. Moreover, since RT-PCR is a platform technology, it will be critical in shaping the national response against future infectious disease pandemics as well. The need of the hour truly is for these response measures to be affordable, accessible and synergistic.
Bhinge is Managing Director, Programmes, Health Initiative, The Rockefeller Foundation and Deo is Professor, Operations Management, Indian School of Business and Executive Director, Max Institute of Healthcare Management, Indian School of Business
Source: Read Full Article